Which Characteristics Describe Most Nonmetals in the Solid Phase
Composed of carbon atoms. They offer basically endless resistance to electric currents.

Metals Nonmetals And Metaliods Crossword Wordmint
3 They are brittle and have metallic luster.

. Compared to a neon atom a helium atom has a 1 smaller radius 2 smaller first. Particles expand to fill any container in which they are placed. In contrast nonmetals are brittle in the solid phase.
Why are most around 90 of elements in their solid phase. Nonmetals display a wide range of chemical properties and reactivities. Describe most non-metals in solid phase.
The electrons in nonmetals are localized in covalent bonds whereas in a metal there is delocalization of the electrons throughout the solid. Theyre malleable and lack metallic luster c. Metals crystallize in closely packed arrays that do not contain molecules or covalent bonds.
Particles are more strongly attached to one another than when in the solid phase. Nonmetals have high ionization energies and electronegativities. Properties commonly seen in nonmetals are.
They are generally poor conductors of heat and electricity. Particles have higher amounts of energy than when in the liquid phase. When metal bond with nonmetal atoms the non metal atoms will.
2 They are malleable and lack metallic luster. Which group 17 element is a solid at room temperature with pressure. Silicon occurs mainly in nature as the oxide and as silicates.
Properties of Nonmetals. The solid form of silicon does not react with oxygen water and most acids. 1 They are malleable and have metallic luster.
High ionization energy and electronegativity. The characteristics of non-metals include brittleness low density and being poor conductor of heat and electricity. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Theyre brittle and have metallic luster d. There are many properties of nonmetals Here are some or most of the properties of nonmetals Easily share or gain valence electron 4-8 electrons in the outer shell 7 for halogens and 8 for noble gases form acidic oxides good oxidizing agents have higher electronegativity may be liquid solid or gas noble gases are gases do not have metallic luster poor conductor. Is this a good thing or a bad thing explain your answer.
And lastly the third class of solids semiconductors has levels of electrical conductivity that are neither very high nor very low. Different structures and different properties. On the opposite side of the spectrum the majority of solid nonmetals are insulators solids whose conductivity is nearly zero.
When combining not metallic atoms metallic atoms generally. Akrypton Bchlorine Cantimony Dmanganese 30Which element is a noble gas. Silicon is a hard relatively inert metalloid and in crystalline form is very brittle with a marked metallic luster.
Then metals are always solids with the exception of mercury in room temperature but nonmetals can be solid liquid or gas. Which characteristic describes most metals in the solid phase. AB Al Ga BC N P CO S Se DSi Ge As 31Which list of elements consists of metalloids only.
4 They are brittle and lack metallic luster. Particles have no motion. This is a characteristic of all metals in the solid phase.
Up to 24 cash back 29Which characteristics describe most nonmetals in the solid phase. Poor conductors of heat and electricity. 1 Describe how the periodic table differentiates between metals and nonmetals Most metals are heavy while almost all nonmental are lighter.
The periodic table organizes elements based on similar physical chemical characteristics. This list of properties is neither essential nor exhaustive. Which characteristic describes most nonmetals the solid phase.
Gain electrons and the resulting inons are larger. Some nonmetals have all of these properties some have very few. Malleable substances can be hammered into a thin sheet.
Metals are generally known to be good conductors of heat and electricity as opposed to non metals that are known to be poor at both. Solid nonmetals are generally brittle with little or no metallic luster. Metals are usually solid at room temperature.
The correct options would be B D and E. Good conductors of electricity. Theyre brittle and lack metallic luster.
Which characteristics describe most nonmetals in the solid phase. 1 good conductors of electricity 2 good conductors of heat 3 malleable 4 brittle. Afirst shell Bsecond shell Cthird shell Dfourth shell 32The valence electrons of a germanium atom in the.
Lose electrons to form posotive ions. Theyre malleable and have metallic luster b. Which characteristic describes most non-metals in the solid phase.
Most nonmetals have the ability to gain electrons easily. Nonmetal structures contain covalent bonds and many nonmetals consist of individual molecules. Which characteristics describe most nonmetals in the solid phase.
Describe the reactivity of elements moving from left group-1 to right group 18.
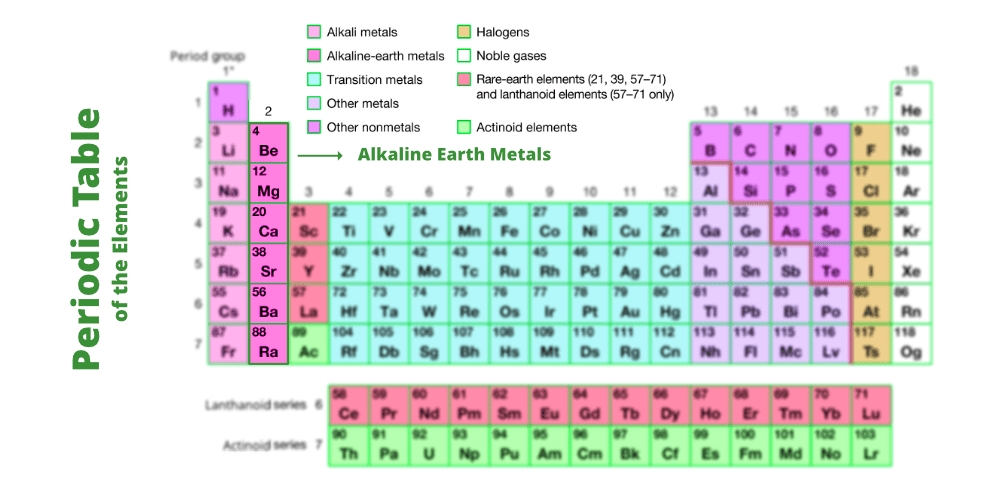
Characteristics Of The Compounds Of Alkaline Earth Metals Geeksforgeeks

States Of Matter Doodle Notes Solid Liquid Gas Plasma Phase Changes Video Video Doodle Notes Doodles Chemistry Lessons

Nonmetals Chemistry For Non Majors
Why Are Metals Shinier And More Lustrous Than Non Metal Elements Quora

Ncert Exemplar Class 8 Science Solutions Chapter 4 Materials Metals And Non Metals

Jmse Free Full Text An Overview Of The Sorption Studies Of Contaminants On Poly Ethylene Terephthalate Microplastics In The Marine Environment Html

Metals Nonmetals And Metaliods Crossword Wordmint

Neon Definition Uses Melting Point Facts Britannica
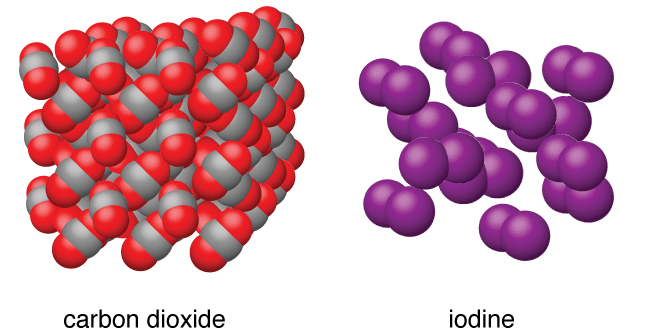
10 5 The Solid State Of Matter Chemistry
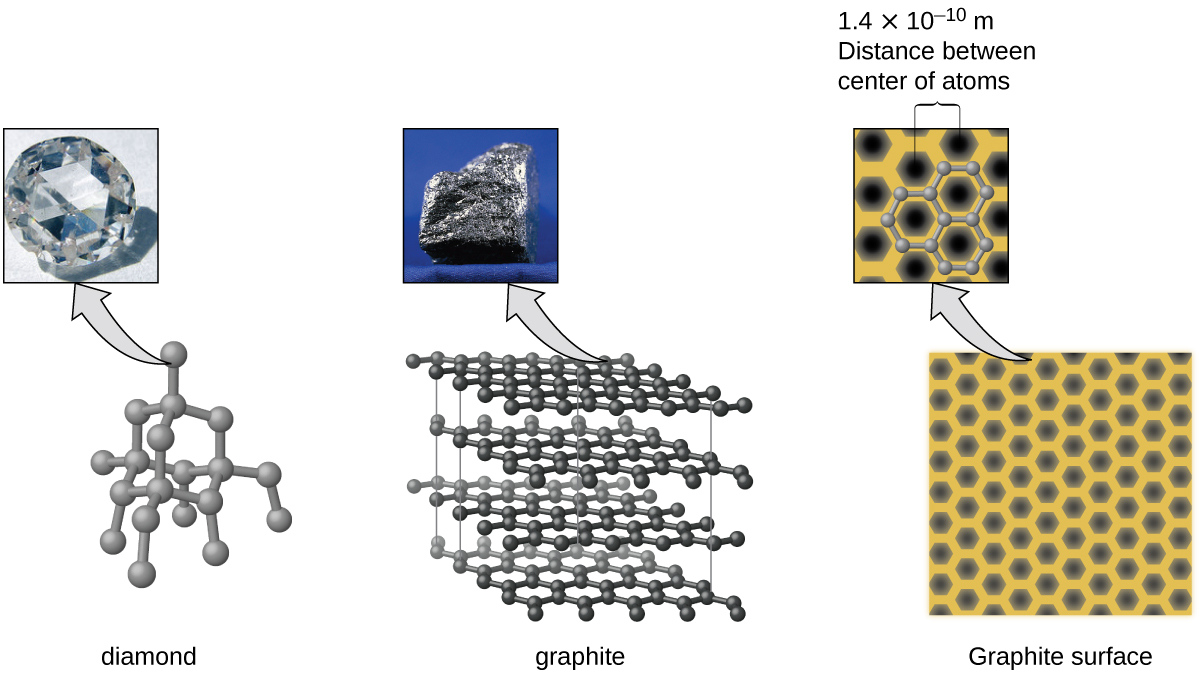
10 5 The Solid State Of Matter Chemistry

States Of Matter Doodle Notes Solid Liquid Gas Plasma Phase Changes Video Video Doodle Notes Doodles Chemistry Lessons
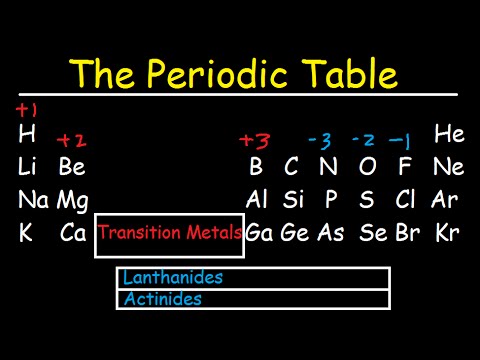
Periodic Table Of Elements Explained Metals Nonmetals Valence Electrons Charges Youtube

Which Characteristics Describe Most Nonmetals In The Solid Phase 1 They Are Malleable And Have Brainly Com

Nonmetals Chemistry For Non Majors
What Is The Difference Between Metal And Nonmetal Quora

Nonmetals Chemistry For Non Majors

Metals Nonmetals And Metaliods Crossword Wordmint

Comments
Post a Comment